Research in our Lab is focused on understanding how cells integrate mechanical cues with growth factor signaling to make fate decisions. We engineer biomaterials and micro-fabricated culture systems both to learn the nature of this integration, and to leverage it for drug discovery, disease modeling and regenerative medicine.
Micro-scale Models of the Human Heart
Genetically defined human in vitro models fulfill an urgent need in developing drugs (for example, to treat cancer) that are not toxic to the adult or developing heart, and in identifying new approaches to treat heart disease. By combining Human Induced Pluripotent Stem Cells (hiPSC) with micro-fabrication and tissue engineering techniques, we form miniaturized “heart muscle in a dish,” in which we can study cell biology and tissue-level physiology (for example, contractile forces). We envision that this in vitro model system will allow us to screen for new medical approaches to treat congenital heart disease, and to identify potentially cardiotoxic drugs early in the drug development pipeline. Currently, we use these miniaturized heart muscles to study genetically-inherited hypertrophic cardiomyopathy, the most common cause of deadly arrhythmias in children. We seek to understand why drugs that are effective in treating non-genetic pathological hypertrophy are less effective in treating this inherited disease.
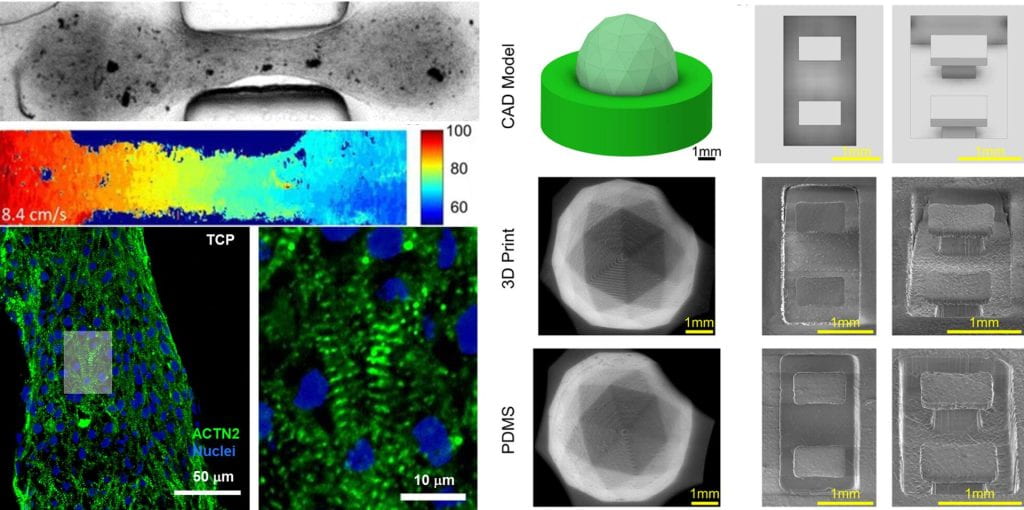
We are especially interested in how biophysical cues, including the extracellular matrix and mechanical strain, affect cardiomyocyte biology. Mechanical stress is a key regulator of cardiomyocyte function, and a number of inherited diseases like hypertrophic cardiomyopathy, along with clinical approaches to treat these diseases, involve beneficial or pathological changes in the stress on individual cells within the heart.
We are investigating how cardiomyocytes respond to changes in mechanical stress with defined engineered tissues based on iPSC. We manipulate mechanical stress using a combination of mechanical cues (tissue geometry, scaffold mechanical properties and external actuation) and genetics. We plan to use these systems to study mechanisms of disease, aide in drug discovery, and direct the design of therapeutic biomaterials. We study cardiomyocyte biology and physiology with biochemical assays, quantitative microscopy and image processing techniques.
Representative publications in this area:
Simmons et al. APL Bioeng. 2024.
Woodhams, Guo et al. Proc. Natl. Acad Sci. USA. 2023.
Simmons et al. ACS Appl. Mater. Interfaces. 2023.
Guo et al. Cell Mol Bioeng. 2021.
Guo et al. ACS Biomater Sci Eng. 2021.
Synthetic Extracellular Matrices
Scaffold-based cell delivery has shown tremendous promise for treating injury and disease. However, control over transplanted cell fate remains challenging. We are developing new approaches to create materials that allow highly localized control over transplanted and endogenous cell fate through the presence of scaffold-bound adhesive signals and growth factors. Our materials function as synthetic mimics of the natural Extracellular Matrix (ECM), which plays a key role in tissue structure and function. We use our ECM mimics to study mechanisms cells use to communicate with and through ECM. We harness this knowledge to design ECM mimics for regenerative medicine applications.
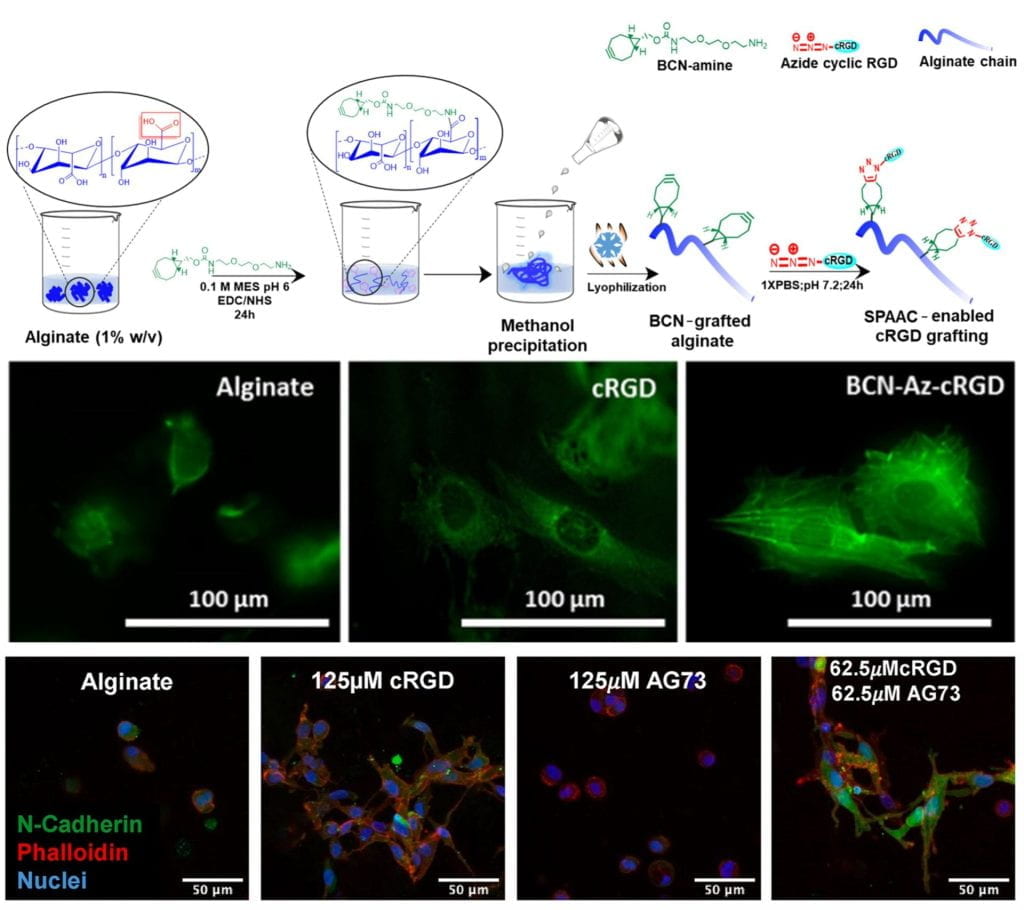
We are especially interested in learning about and exploiting how mechanical properties of ECM (including elasticity and porosity) affect cell fate. The ECM plays an important role not only in signaling through adhesion receptors (e.g. integrins) but also by acting as a local depot for growth factors, and we are also interested in how binding to ECM affects growth factor bio-activity. We are using orthogonal click chemistries to independently control hydrogel-presentation of ECM and growth factor mimicking peptides, to study how these different signals synergize to control cell fate. We study cell behavior with biochemical approaches and quantitative microscopy.
Representative publications in this area:
Tan et al. Biomaterials. 2021.